

BIOAPPS. is founded, promoted and led by team of technocrats and professionals who have been serving the Analytical, Life Science and Biotech industry for over two decades.....
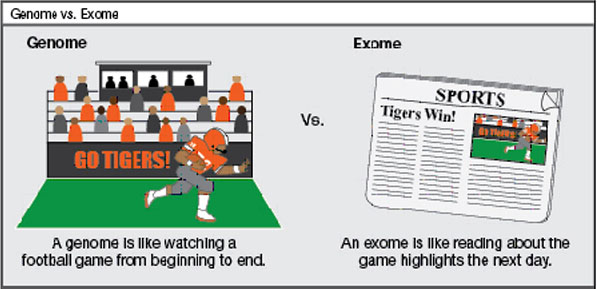
Your DNA is your own personal blueprint for life. The genome includes a person’s entire DNA, both the introns and the exons. The exome includes only the exons (the parts used to make proteins). The introns and other non-coding sequences of DNA are not part of the exome. If you think of the genome as all of the action in a football game, the exome is like the game highlights with many of the important plays.
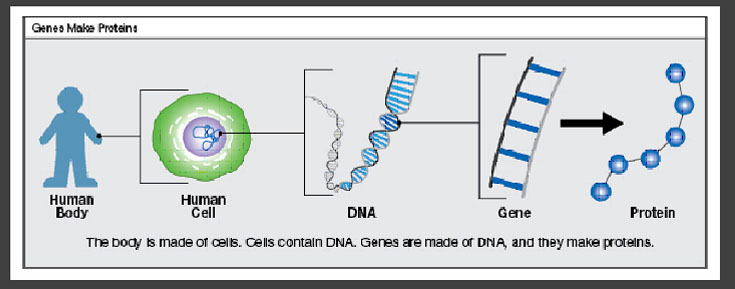
Genes are chemical instructions that tell a cell what job to perform. A gene is like a recipe for making a particular protein. Proteins do important jobs in every cell of the body.
Genes are written in code, with four chemicals (represented by the letters A, T, C, and G) that spell out the instructions to make a protein. People generally have two copies of most genes, one copy from each parent.
Your DNA is your own personal blueprint for life. The genome includes a person’s entire DNA, both the introns and the exons. The exome includes only the exons (the parts used to make proteins). The introns and other non-coding sequences of DNA are not part of the exome. If you think of the genome as all of the action in a football game, the exome is like the game highlights with many of the important plays.
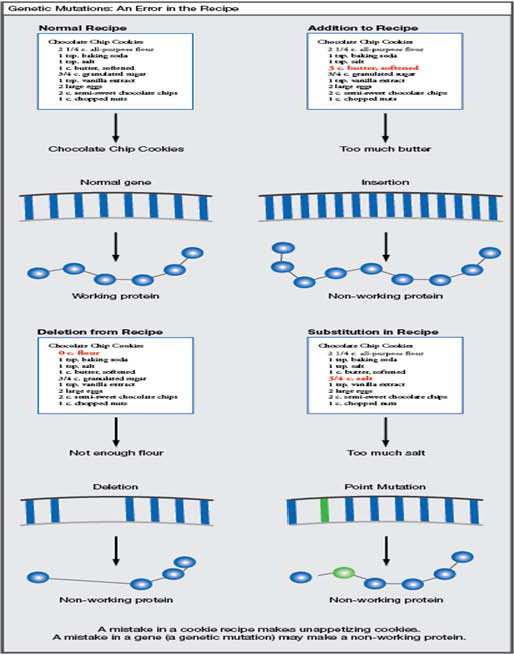
A mutation is a change in the DNA. Mutations may cause proteins to work poorly or to not be made at all. If you think of a gene as a recipe for making a protein, a mutation is like a mistake in the recipe that causes the protein to not work normally.
Mutations may be “misspellings” where the wrong letter is included in the gene, or they may be deletions or duplications where there is missing or extra DNA in a gene. Genetic mutations are random. People cannot do anything to cause mutations or to stop them from happening.
Exome sequencing is a targeted sequencing approach that is restricted to the protein-coding regions of genomes. The exome is estimated to encompass approximately 1% of the genome, yet contains approximately 85% of disease-causing mutations . For genetic researchers trying to identify the genes implicated in over 6,800 rare diseases , exome sequencing enables the identification of common single nucleotide variants (SNVs), copy number variations (CNVs), and small insertions or deletions (indels), as well as rare de novo mutations that may explain the heritability of Mendelian and complex disorders .
Copy number variations (CNVs) represent a class of genomic variation in which large regions of the genome (>1 kb) are duplicated or deleted. Until recently, researchers mainly employed methods such as array comparative genomic hybridization (aCGH) or fluorescence in situ hybridization (FISH) to detect CNVs and other types of genomic alterations. However, microarray-based methods are limited by the requirement of array design, have limited dynamic range compared with other methods, and depending on array design, only gross-level changes can be detected while smaller variations might be missed. It not only allows discovery of novel copy number variants but also provides important genetic information such as single-nucleotide variants (SNVs) and small insertion/deletions (indels) that link variants to biological pathways found in disease research. Coupled with specific CNV workflows within the Ion Reporter Software, the kit enables simultaneous identification of SNVs, small indels, and now, gene-level CNVs up to aneuploidies within one sample in a single day.
The causes of most Mendelian disorders, or single-gene disorders, have been found in the exonic regions of the causative gene. Traditional approaches such as linkage mapping and Sanger sequencing of candidate genes have contributed to the discovery of causative variants for Mendelian disorders. However, these approaches are often costly or time-consuming, or have limited power due to small sample sizes. Through affected archived sample trio analysis, exome sequencing can be a powerful approach for identifying the causative variation responsible for Mendelian disorders .
Genome-wide association studies (GWAS) have identified a large number of variants that contribute to heritability of complex traits. These studies often have challenges in differentiating the functional consequences of the identified common variants, and in most studies the loci identified only partially explain heritability—this has become known as the “missing heritability” of common or complex multigenic disorders. The role of rare alleles in the heritability of complex disorders is not fully understood. To this end, it enables accurate detection of rare and common variants in the protein-coding regions of the genome, making it a costeffective method for elucidating the causes of complex disorders in your research. The detection and discovery of unknown variants in multiple samples reduces total cost and time, enabling us to gain comprehensive insight into complex disorders.
Exome sequencing enables cancer researchers to detect germline genetic alterations that may predispose cells to cancer in the future. The goal of sequencing the exome is to identify coding variants and mutations critical in the development of tumors. It enables us to focus on the protein-coding regions of genomes to identify the mutations, deletions, or copy number variations that can help provide important insights into tumor genetic pathways. It offers a simple and flexible workflow that targets ~33 Mb of coding exons— greater than 97% of coding regions as described by consensus coding sequence (CCDS) annotation. It offers the advantages of low DNA input, with as little as 50 ng required for each individual library.